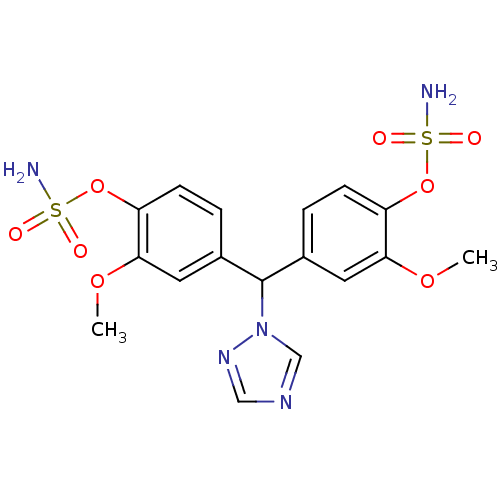
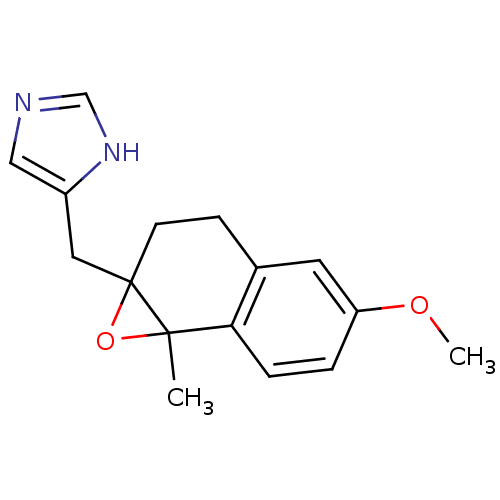
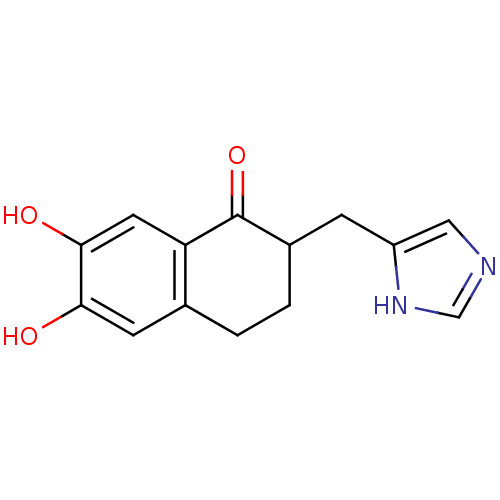
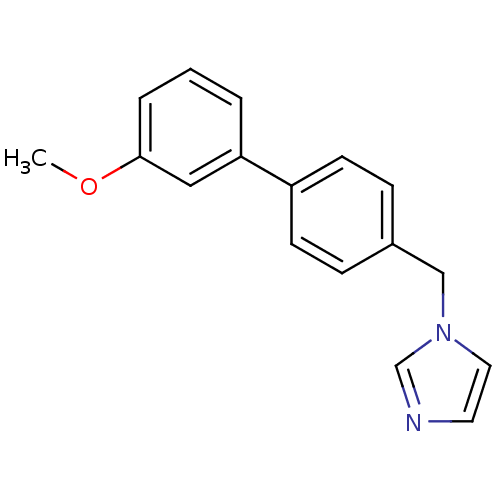
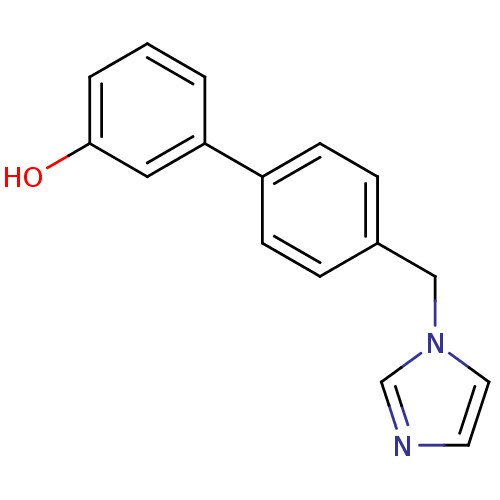
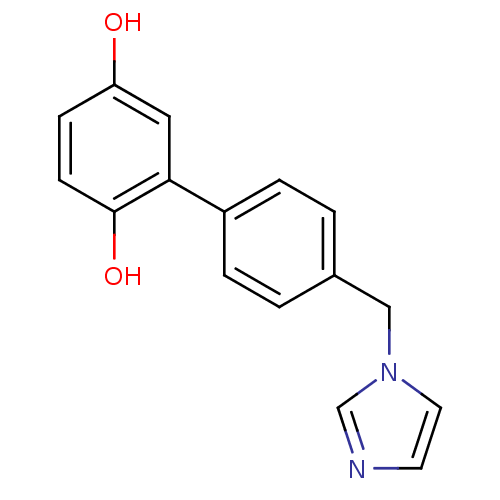
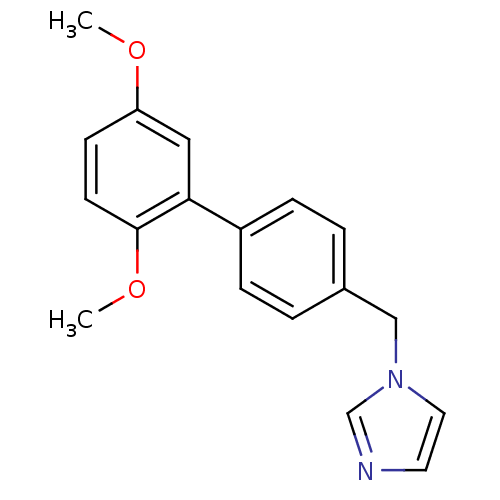
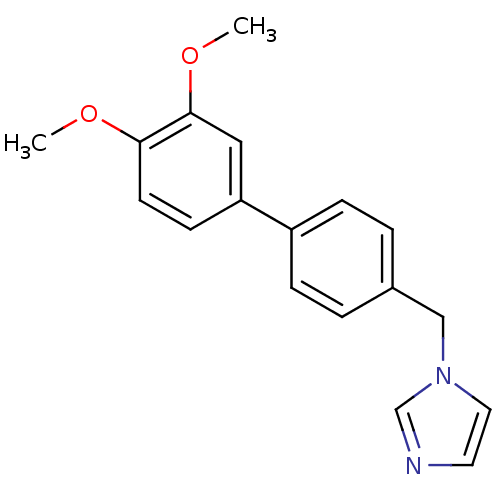
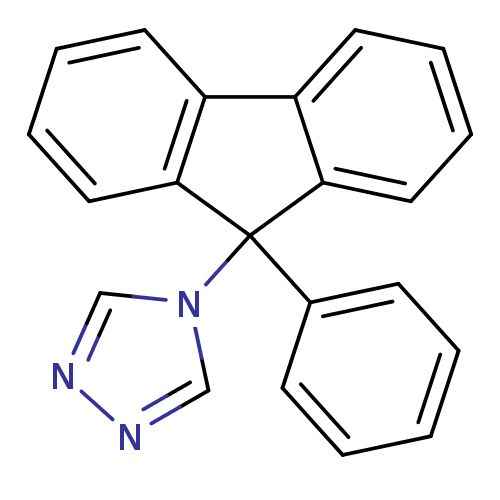
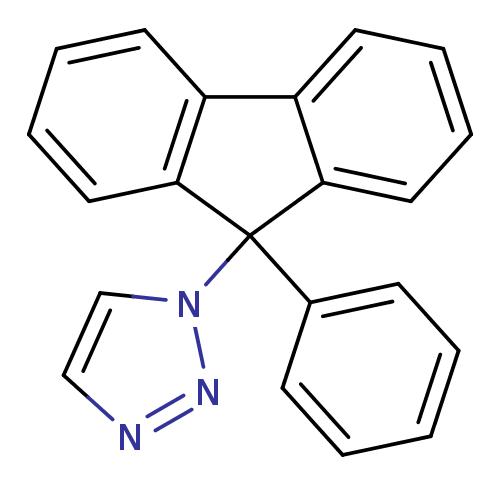
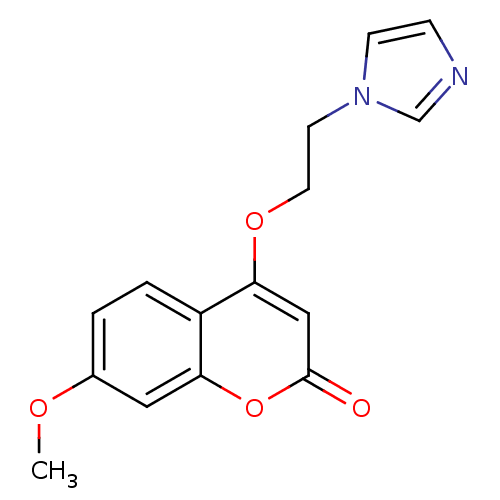
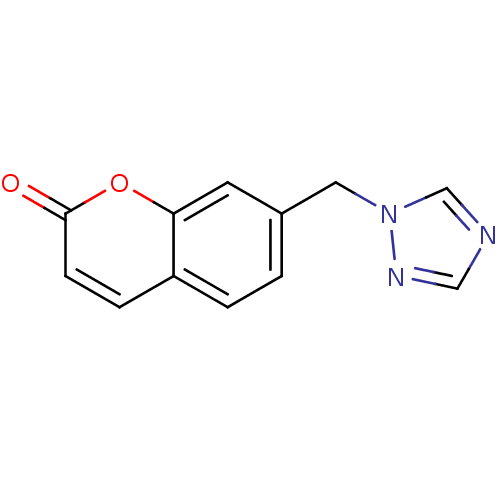
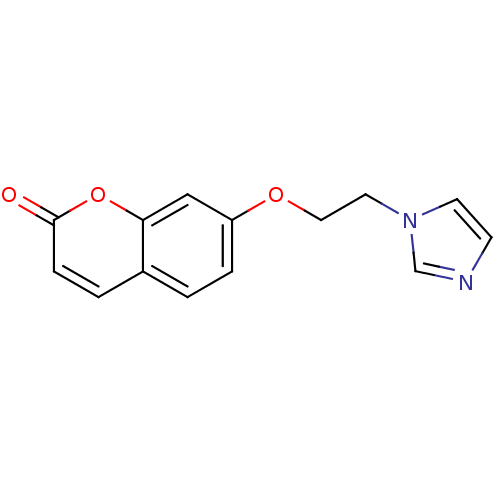
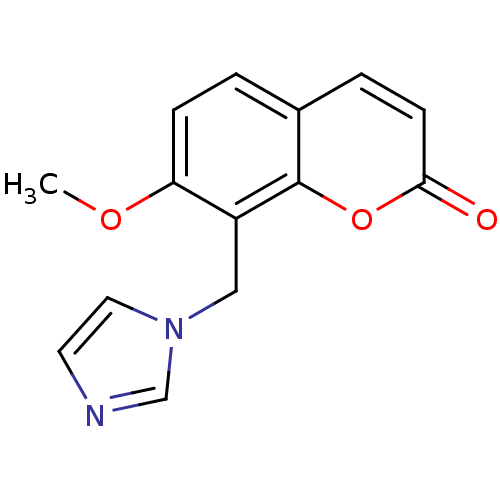
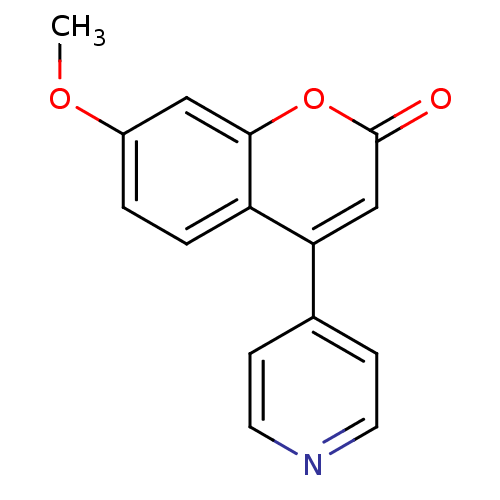
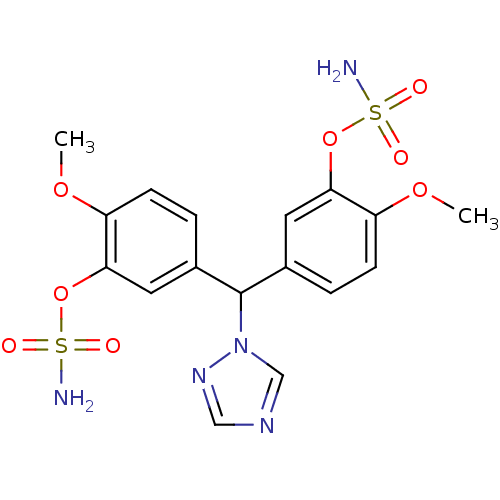
Affinity DatapH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DatapH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.4 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma...More data for this Ligand-Target Pair
Affinity DatapH: 7.5 T: 2°CAssay Description:The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ...More data for this Ligand-Target Pair
Affinity DataAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea...More data for this Ligand-Target Pair